I knew that I needed to do something.
The supper dishes were done, and a beautifully empty and warm evening was stretching out ahead of me, but I was itching, with a mounting frenetic energy that was off-gassing from my anxious mind.
At first, I thought about going for a walk around the neighborhood, or, doing double duty, grabbing my camera and heading down the road to a nearby baseball field. There, atop one of the warm outfield floodlights, a pair of osprey had built their massive nest, and were tending to their growing, comically demanding, only chick.
Neither of these ideas felt quite right, though.
Looking outside to our yard, my husband Ben then suggests we deal with the detritus gathered earlier in the summer from our yard work: a nest of trimmed cedar branches, which once snaked through underbrush and choked out nearby lilacs, and the remains of a rotting deck, torn off the house and relegated to kindling once we were sure it wasn’t pressure-treated.
We light a match, and begin.
A fire is a fascinating chemical reaction.
Friction creates heat. When applied to a fuel source, like decaying 2x4s or errant brush, it sparks, then creates a tiny flame that hopefully grows in size as it dances along the length of the logs and heats the wood to higher temperatures still.
Any water remaining within the wood fibres quickly turns to vapour. If there’s too much water in the wood, the temperature plateaus and the fire is stifled. But, if it’s dry enough, the wood reaches a high enough temperature that it can begin to pyrolyse, or literally, break down by fire. The light grows brighter still. The wood decomposes, turns black and leaves ash, as the carbon bonds break apart, only to bond with the oxygen in the surrounding air.
Because it’s not an infinite process - fuel can only produce so much carbon for oxidation - over the course of four hours, we methodically cut branches and boards into like-size piles, and feed the growing mound of embers. A stash of dryer lint and paper towel tubes - more delicious carbon - speeds up the initial preheating process.
Providing a light to combat the darkening skies, I pause at times in feeding the flames to take in the deep orange glow. The flames flicker and violently lick at the air, before sending swirling sparks up towards the young maples overhanging the pit.
It takes me longer than it feels it should to explain this reaction, but even “simple” science can get bogged down with jargon and assumptions.
A young Alan Alda once asked his teacher what fire was, and was given a one-word explanation (“Oxidation”), as if that was any clearer. This non-answer became a sticking point. Some fifty years later, through the Alda Center, he launched the “Flame Challenge”.


What’s fascinating is that, although the Flame Challenge speaks to the idea of sharing ideas in plain, clear words without using more jargon, the kids judging the submissions preferred an explanation that didn’t go “too simple”.
They want to learn. They just need us to be clear about it.
I consider possible definitions of “fire” that make sense. My attempt above is better than previous versions I’ve created in my head, but can still use improvement. Meanwhile, the sharp edges present earlier in my mind have gradually smoothed out as the evening continues, revealing an underlying fatigue.
In my tired state, I get eager to make more headway in the pile of debris faster, and as a result lose focus; too many damp leaves are placed on the fire. I thought it was hot enough to withstand it; instead, thick waves of opaque smoke unfurl, stifle the heat, make me cough. I remind myself: Slow down. Don’t rush and overwhelm what you have to give.
I grab a metal rake and push around the leaves, coaxing more oxygen around the fuel to encourage combustion. Time heals, and the fire builds up again.





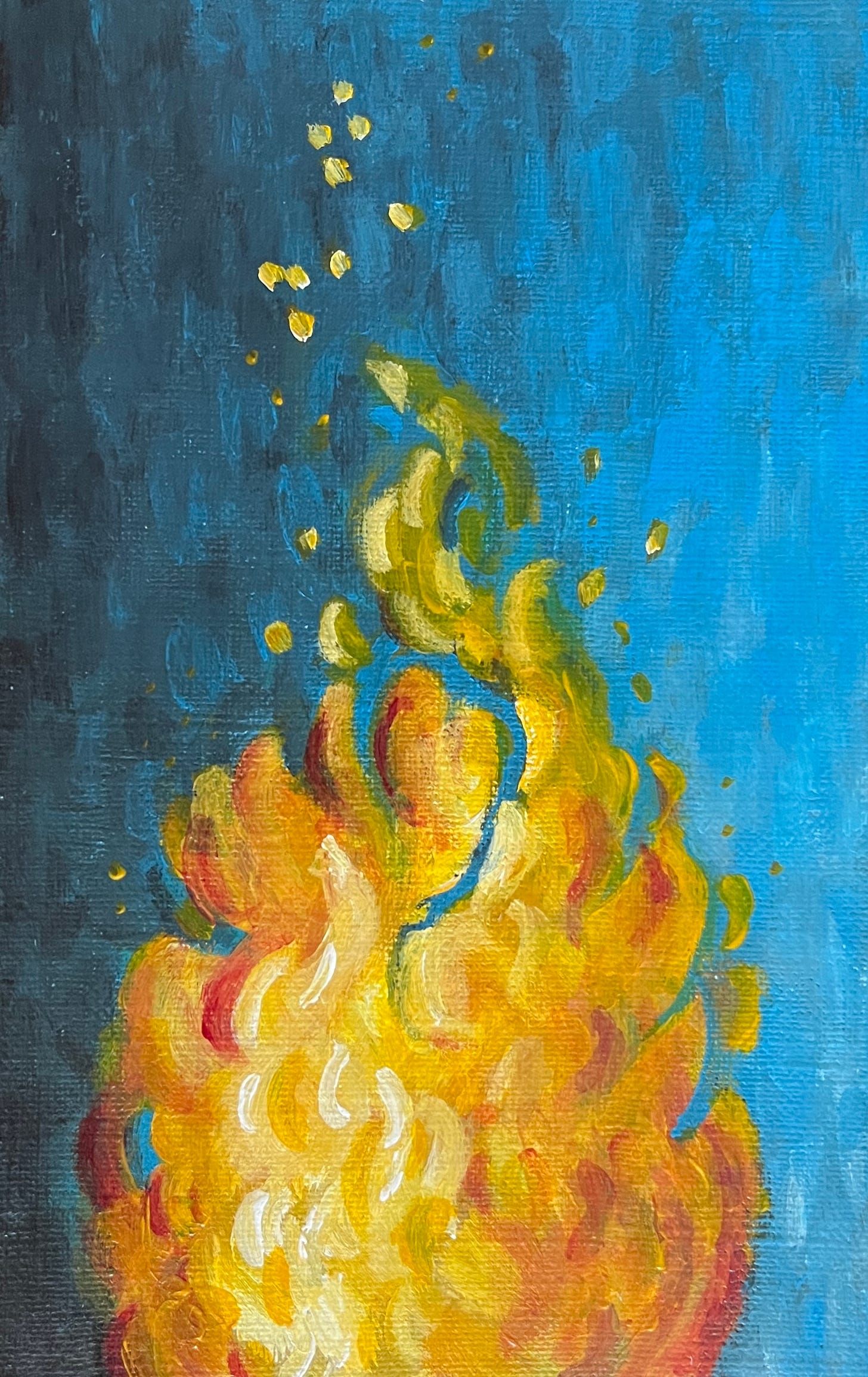

Ah, another great one. "Don't rush and overwhelm what you have to give." So true in several senses.
Another fascinating post. I love the painting!